ProCvix helps identify and stratify cervical cancer risk, for women with HPV.
High-sensitivity screening test targeting E6/E7 DNA of 14 high-risk HPV types for improved risk stratification, and higher specificity for triage and disease progression.
Simultaneously detects and differentiates all high-risk types including 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68.
Easy to use, with a single, multiplex, real-time PCR reaction.
Assay Details
Target sequence: E6/E7 region
Limit of detection: 100 copies for all HPV types
Quality Control: Positive and internal cellular controls of PCR amplification and sample integrity
Validated sample type: Cervical (ThinPrep)
Protean’s ProCvix report provides the information you need in a clear and easy to understand format.
High risk HPV screening result status (normal vs abnormal)
Cervical cancer screening result (normal vs abnormal)
High risk HPV findings
Oncogenic E6/E7 mRNA result
Ordering Process:
Complete the test requisition form
Ship Sample Collection Kit to Protean’s laboratory with completed TRF
Receive ProCvix results within 3-5 business days
Sample Requirements:
Liquid Based Cytology (LBC) samples conserved in PreservCyt (ThinPrep®, Hologic) and Cyt-All (Cytomega) transport media
OR; Urine Sample collected with Colli-Pee device
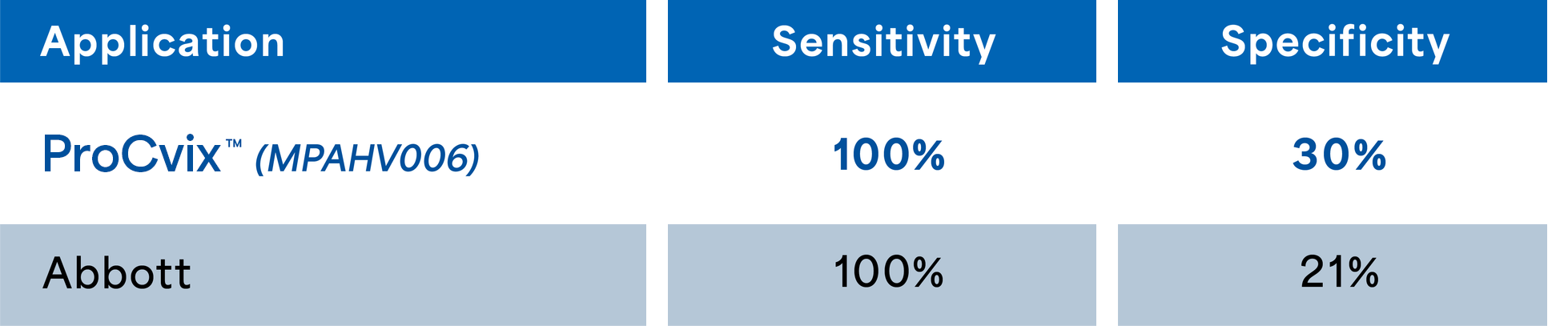
Specificity & Sensitivity for ProCvix
Please Note: ProcVix is a 2-step in vitro qualitative multiplex real-time PCR test.
ProcVix is intended as a high-sensitivity screening assay. Lower specificity for cervical intraepithelial neoplasia is an expected and accepted trade-off in screening settings, where positive results are followed by confirmatory testing.

Order ProCvix™
Fill out your name and email address below and someone from Protean’s support team will be in touch shortly.
References
Cuschieri K, Latsuzbaia A, McMahon H, et al. Clinical performance assessment of the Papilloplex HR-HPV assay on self-taken urine and vaginal swab samples: findings from a multicentre European study. J Clin Pathol. Published online November 23, 2025. doi:10.1136/jcp-2025-210211
Bhatia R, Alcaniz Boada E, Bonde JH, Quint WGV, Xu L, Ejegod DM, Cuschieri K, Arbyn M. Papilloplex HR-HPV test has non-inferior clinical performance for detection of human papillomavirus infection: assessment using the VALGENT framework. J Clin Pathol. 2023;76(3):172–176. doi:10.1136/jclinpath-2021-207864
Bhatia R, Serrano I, Wennington H, Graham C, Cubie H, Boland E, Fu G, Cuschieri K. Evaluation of a novel single-tube method for extended genotyping of human papillomavirus. J Clin Microbiol. 2018;56(1):e01687-17. doi:10.1128/JCM.01687-17
Bhatia R, Alcaniz Boada E, Bonde JH, et al. [Article in press] J Clin Pathol. Epub ahead of print, April 1, 2025. doi:10.1136/jclinpath-2021-207864
Giubbi C, Martinelli M, Vallini I, et al. Human papillomavirus (HPV) detection in vaginal self-samples: evaluation of eNat® as an alternative suspension medium to ThinPrep® PreservCyt® for vaginal swabs. Open Research Europe. 2022;2:35. doi:10.12688/openreseurope.14344.2