An elevated PSA score doesn't always mean cancer.
ClarityDX helps you to make informed deicisions after receiving an elevated PSA result.
The issue is that although higher PSA levels can indicate prostate cancer, they can also be caused by vigorous exercise, recent sexual activity, a prostate infection, and more. In fact, studies show that up to 80% of men with elevated PSA level have low risk of prostate cancer, or no prostate cancer. [2] This may lead to unnecessary invasive prostate biopsies and treatments with potentially harmful side effects.
ClarityDX Prostate is a fast, accessible, clinically-proven decision support tool that combines multiple data points, including PSA levels, and uses the Clarity machine learning platform to calculate a more accurate prostate cancer risk score.
Clinical data shows that adding ClarityDX Prostate to the patient care pathway could reduce unnecessary prostate biopsies by up to 47%. [5]
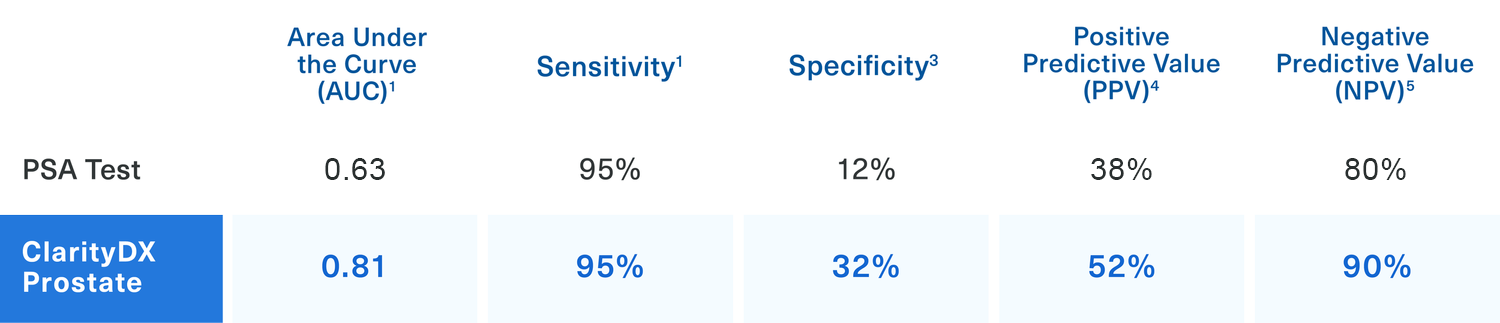
ClarityDX Prostate Outperforms Standard PSA Testing
Note: Area Under the Curve is used as a quantitative measurement of the accuracy of the diagnostic test.
ClarityDX Prostate helps patients and their physicians make informed treatment decisions after receiving an elevated PSA test result.

Key Features of ClarityDX Prostate
Non-invasive and easily performed blood test that determines the risk of prostate cancer 4x more accurately than PSA alone.
Provides a risk score based on clinical and biological biomarkers, to help make informed decisions after elevated PSA test result.
Reduces unnecessary biopsies and harmful side effects by up to 47%.
Highly accurate and clinically proven diagnostic tool that utilizes machine learning.
Who is Eligible for ClarityDX Prostate?
Men with clinical suspicion of prostate cancer (elevated PSA, abnormal DRE, or symptoms)
Does not have a prior diagnosis of prostate cancer.
Ordering Process:
Complete the Cancer Screening Test Requisition Form
Send the completed test requisition form via fax or Protean’s secure electronic portal, or include with Sample Collection Kit
Ship required serum samples to Protean’s laboratory
Receive results within 3-5 business days
Sample Requirements:
Blood sample, spun down
2 SST serum tubes
Stable for 5 days at 2 to 8 °C
Stable for 24 hours at 20-25 °C
Freeze at -20 °C or refrigerate at 2 to 8 °C immediately after blood is spun down*
*Storage temperature is site specific
Order ClarityDX Prostate Today
Fill out your contact information below, and we will reach out to you with next steps.
Questions about ClarityDX Prostate?
Call us at: 1 (754) 242 9682 | Email us at: info@proteanbiodx.com

ADDITIONAL NOTES
Reference values: the test demonstrated an area under the receiver operating characteristic curve (AUC) of 0.80 to 0.87 and a sensitivity of 0.95 and specificity of 0.32 to 0.47, when using a Risk Score threshold of 17% or 25%, depending on the information available and the ClarityDX Prostate model used.
Performance characteristics : The performance characteristics of ClarityDX Prostate® were determined by Nanostics in a population 40 to 75 years of age with PSA ≥ 3 ng/mL. Evaluation of this test outside of these ages and PSA values has not been performed by Nanostics. Total PSA and free PSA tests are indicated for men ≥ 50 years of age; caution is required when interpreting individual total PSA and free PSA results in patients below 50 years of age. Patient management should be based on holistic clinical judgment. This test has not been cleared or approved by the U.S. Food and Drug Administration (FDA) or Health Canada.
CITATIONS
[1] Wallis et al. (2023).JCO 41, 5023-5023. 10.1200/JCO.2023.41.16_suppl.5023
[2] Moses et al., NCCN Guidelines, V1.2023. https://doi.org/10.6004/jnccn.2023.0014
[3] Bell, et al. CMAJ Nov 2014 186 (16) 1225-1234; DOI: 10.1503/cmaj.140703
[4] Paproski et al. (2023). Eur Urol 83, S91-S92. https://doi.org/10.1016/S0302-2838(23)00119-7
[5] Paproski et al. (2023). Can Urol Assoc J. 17 ((6 Suppl 2)), S117–S118. doi: 10.5489/cuaj.8417